Informational
Electron Affinity: A Complete Guide to Trends, Importance, and Applications
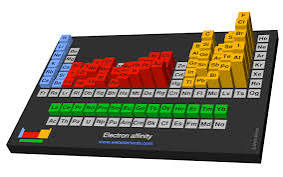
Electron Affinity: A Complete Guide to Trends, Importance, and Applications
Introduction to Electron Affinity
The concept of electron affinity is one of the most important topics in chemistry that helps explain how elements behave when gaining electrons. In simple terms, electron affinity refers to the amount of energy released when a neutral atom gains an extra electron to form a negatively charged ion. This property provides valuable insights into the reactivity of elements, their placement in the periodic table trends, and their relation with other fundamental concepts like ionization energy, electronegativity, and electron configuration.
Understanding electron affinity allows scientists, students, and researchers to predict chemical bonding, molecular structures, and even biological interactions at the atomic level. In this article, we will cover everything about electron affinity, including definitions, periodic trends, factors affecting it, comparisons with related properties, and practical applications.
What is Electron Affinity?
Electron affinity can be defined as the energy change that occurs when one mole of neutral atoms in the gaseous state each gain an electron to form a mole of negative ions. For example:
C
l
(
g
)
+
e
−
→
C
l
−
(
g
)
+
E
n
e
r
g
y
Cl(g)+e
−
→Cl
−
(g)+Energy
In this reaction, chlorine releases energy when it gains an electron. This released energy represents its electron affinity.
Key points about electron affinity:
It is usually measured in kilojoules per mole (kJ/mol).
A higher value of electron affinity indicates that the atom strongly attracts additional electrons.
Nonmetals, particularly halogens, tend to have higher electron affinity values compared to metals.
Factors Affecting Electron Affinity
Several factors influence the electron affinity of an element. The most significant ones include:
- Atomic Radius
Smaller atoms with a smaller atomic radius tend to have higher electron affinity values because the added electron is closer to the nucleus and feels stronger attraction. - Nuclear Charge
Elements with higher nuclear charge (more protons in the nucleus) usually exhibit greater electron affinity, as the nucleus strongly attracts the incoming electron. - Electron Configuration
The stability of an atom’s electron configuration plays a major role. Atoms that achieve stable noble gas configurations upon gaining an electron have higher electron affinity. For example, chlorine has high electron affinity because it becomes stable with a full outer shell. - Subshell Occupancy
The position of an atom in the periodic table trends also matters. Adding an electron to a half-filled or fully filled subshell requires more energy, leading to lower electron affinity values.
Periodic Table Trends in Electron Affinity
The periodic table trends clearly demonstrate how electron affinity changes across periods and groups:
Across a Period (Left to Right)
Electron affinity generally increases across a period in the periodic table trends.
This happens because the atomic radius decreases while the nuclear charge increases, allowing the nucleus to attract extra electrons more strongly.
For example, halogens like fluorine and chlorine show very high electron affinity values.
Down a Group (Top to Bottom)
Electron affinity usually decreases down a group.
The atomic radius increases, meaning the new electron is added farther from the nucleus, reducing the attraction.
For instance, chlorine has higher electron affinity compared to iodine.
Relationship Between Electron Affinity and Other Properties
- Electron Affinity vs. Ionization Energy
While electron affinity measures the energy released when an atom gains an electron, ionization energy refers to the energy required to remove an electron from a neutral atom. Both properties are closely linked in explaining reactivity.
Atoms with high ionization energy and high electron affinity (like fluorine) are highly reactive nonmetals.
Metals typically have low ionization energy and low electron affinity, which explains their tendency to lose rather than gain electrons.
- Electron Affinity vs. Electronegativity
Electronegativity is a measure of an atom’s ability to attract shared electrons in a chemical bond. Although related, it is different from electron affinity:
Electron affinity is an energy change for isolated atoms.
Electronegativity deals with bonded atoms within molecules.
However, atoms with high electron affinity usually also have high electronegativity.
- Electron Affinity and Atomic Radius
As mentioned earlier, smaller atomic radius values correlate with higher electron affinity, since the incoming electron is closer to the nucleus.
High Electron Affinity Elements
Some elements are particularly notable for their high electron affinity values:
Halogens (Fluorine, Chlorine, Bromine, Iodine) – Halogens are at the top because they need only one electron to complete their octet.
Oxygen and Sulfur – Though not as high as halogens, these elements also have relatively high electron affinity.
Noble Gases – These elements typically have almost no electron affinity because their electron configuration is already stable.
Practical Applications of Electron Affinity
Understanding electron affinity has real-world applications in many areas of science and technology:
Predicting Reactivity
Electron affinity helps explain why halogens are highly reactive and why noble gases are chemically inert.
Chemical Bonding
Electron affinity combined with electronegativity provides insights into ionic and covalent bond formation.
Material Science
Semiconductor design often relies on electron affinity to determine how easily electrons can move in materials.
Biological Chemistry
In biological systems, electron affinity plays a role in processes like electron transport chains and redox reactions.
Environmental Chemistry
Predicting pollutant interactions with natural systems often involves considering their electron affinity.
Common Misconceptions About Electron Affinity
Electron affinity is not always positive: While most nonmetals release energy when gaining an electron, some elements may require energy input (negative values).
Electron affinity is different from electronegativity: Students often confuse these terms, but one deals with isolated atoms, the other with bonded atoms.
Electron affinity does not always follow a perfect trend: While general periodic table trends exist, there are exceptions due to unique electron configuration patterns.
Summary
Electron Affinity Central concept explaining energy changes when atoms gain electrons.
Electron Configuration Determines stability and influences affinity values.
Ionization Energy The opposite process, helps compare reactivity.
Periodic Table Trends Show how electron affinity increases across periods and decreases down groups.
Electronegativity Related but distinct property measuring attraction in bonds.
Atomic Radius Directly impacts how strongly an atom attracts extra electrons.
Conclusion
In conclusion, electron affinity is a key chemical property that explains why some elements readily gain electrons while others resist. By analyzing periodic table trends, electron configuration, ionization energy, atomic radius, and electronegativity, we gain a complete understanding of atomic behavior.
From predicting chemical bonding to designing new materials, the concept of electron affinity plays a central role in modern chemistry. Whether you are a student, teacher, or researcher, mastering this topic will give you deeper insights into the building blocks of matter and their interactions.

GK
How Do Satellites Stay in Orbit? A Complete Guide

How Do Satellites Stay in Orbit? A Complete Guide
When we look up at the night sky, we often wonder, how do satellites stay in orbit? These human-made marvels play a crucial role in communication, weather forecasting, navigation, and scientific research. But the science behind their motion often sparks curiosity. In this blog, we will explore how do satellites stay in orbit, how do satellites move, how do satellites work, and how do satellites orbit the Earth in a simple, easy-to-understand way.
The Basics: How Do Satellites Stay in Orbit?
To answer the big question—how do satellites stay in orbit—we need to understand gravity and velocity. A satellite is launched into space with enough speed to counteract the pull of Earth’s gravity. If it moves too slowly, it will fall back to Earth. If it moves too quickly, it will escape Earth’s gravity. The perfect balance between speed and gravity is what allows satellites to remain in orbit.
This balance is why the question of how do satellites stay in orbit fascinates scientists and space enthusiasts alike. Simply put, satellites are constantly “falling” toward Earth due to gravity, but because they are moving sideways so quickly, they keep missing Earth, creating a continuous orbit.
How Do Satellites Move in Space?
Now that we understand how do satellites stay in orbit, another question arises: how do satellites move?
Satellites move in space because of the momentum given to them during launch. Once in orbit, they don’t need engines running continuously to keep moving. Instead, they rely on the laws of physics—specifically Newton’s First Law of Motion, which states that an object in motion stays in motion unless acted upon by an external force.
So, how do satellites move? They circle Earth at extremely high speeds—often thousands of kilometers per hour. This movement ensures that they maintain their orbit while serving their purposes, such as GPS navigation, weather monitoring, or internet connectivity.
How Do Satellites Work?
Understanding how do satellites work helps us appreciate their role in daily life.
Satellites are equipped with communication systems, sensors, solar panels, and antennas. Depending on their type, they collect data, send signals, or relay communication. For example, weather satellites take pictures of cloud formations, while GPS satellites send signals that help your phone calculate your location.
So, how do satellites work in practice? They receive signals from Earth, process them, and send them back, sometimes bouncing information between multiple satellites to ensure coverage across the globe. Without satellites, modern life—from weather reports to smartphone maps—would look very different.
How Do Satellites Orbit the Earth?
Another important concept is how do satellites orbit the Earth. When a satellite is launched, it’s placed into a specific orbit depending on its mission.
So, how do satellites orbit the Earth effectively? They follow paths determined by their speed, altitude, and Earth’s gravitational pull. Low Earth orbit (LEO) satellites, for example, orbit closer to Earth and are used for imaging and communication. Geostationary satellites orbit much farther away, appearing “fixed” over one point of Earth, which makes them perfect for weather and television broadcasting.
Understanding how do satellites orbit the Earth reveals the planning and precision behind every satellite launch. Engineers calculate exact speeds and angles to ensure satellites serve their purpose without drifting off course.
Everyday Examples of Satellites in Action
Communication satellites: Deliver TV, radio, and internet services.
Weather satellites: Help meteorologists forecast storms and track climate patterns.
GPS satellites: Guide airplanes, ships, cars, and smartphones.
Scientific satellites: Observe Earth, study space, and provide crucial research data.
In all these cases, the core principles of how do satellites stay in orbit, how do satellites move, how do satellites work, and how do satellites orbit the Earth are at play.
Why Understanding Satellites Matters
Knowing how do satellites stay in orbit isn’t just for space scientists—it also helps us appreciate the technology we use every day. Whether you’re checking weather updates, navigating with GPS, or streaming a live event, satellites are silently working above us.
Understanding how do satellites move gives insight into their reliability. Recognizing how do satellites work highlights their contribution to global communication. And exploring how do satellites orbit the Earth shows how human innovation has harnessed natural forces to achieve incredible technological feats.
Final Thoughts
So, how do satellites stay in orbit? They balance the pull of Earth’s gravity with their speed, creating a stable path around our planet.
How do satellites move? By maintaining momentum once launched into space.
How do satellites work? Through advanced systems that collect and transmit signals and data.
And finally, how do satellites orbit the Earth? By following carefully calculated paths that match their mission requirements.
Satellites are not just objects floating in the sky—they are the backbone of our modern, connected world.

GK
Why Do Ants Communicate? Secrets of Their Tiny World

Why Do Ants Communicate? Secrets of Their Tiny World
When you see a line of ants moving in perfect coordination, you might wonder: Why do ants communicate so effectively compared to other insects? Ants are social creatures that thrive in colonies, and their survival depends on constant communication. Understanding why do ants communicate opens up fascinating insights into nature’s most cooperative societies.
Why Do Ants Communicate?
Ants live in large colonies where cooperation is key. From finding food to protecting their queen, ants rely on teamwork. So, why do ants communicate so much? The answer is simple: they need to share vital information. Without communication, their organized system would collapse.
But why do ants talk to each other in ways humans can’t easily see? They use chemical signals, body language, and even touch. These methods help ants build trails, warn others about danger, and decide who does what job in the colony.
What Do Ants Communicate With Each Other?
One major question scientists ask is: what do ants communicate with each other? Ants mainly communicate through pheromones—special chemicals they release into the air or onto the ground. For example, when a worker ant finds food, it leaves a scent trail so others can follow.
Another answer to what do ants communicate with each other includes danger signals. If predators threaten the colony, ants release alarm pheromones, and instantly, the army of ants prepares to defend their home.
So, why do ants communicate? Because sharing food sources, defense strategies, and even instructions about colony building is essential to their survival.
Why Do Ants Talk to Each Other?
If you’ve ever watched ants closely, you might think: why do ants talk to each other when they can simply work on their own? The truth is, no single ant could survive long without help. Ant colonies are like super-organisms, and each ant is just one small part of the larger system.
Scientists discovered that ants talk to each other not only with chemicals but also by tapping their antennae. This is how they pass on messages about food, danger, or even grooming needs.
So, why do ants talk to each other so often? Because it keeps the colony strong, united, and efficient.
What Do Ants Talk About?
Have you ever wondered: what do ants talk about during their busy lives? While ants don’t talk the way humans do, their signals carry important “conversations.” For example, what do ants talk about includes:
Food locations
Predators or threats
Which direction to travel
Nest maintenance
Caring for larvae and the queen
So, if you ask, what do ants talk about, the answer is survival and teamwork. Every message strengthens the colony’s unity.
What Do Ants Use to Communicate?
Now let’s answer another vital question: what do ants use to communicate? The main tools are:
Pheromones – chemical signals to mark paths and warn of threats.
Antennae tapping – a form of “touch language.”
Vibrations – some ants can produce sound by rubbing body parts, known as stridulation.
Therefore, what do ants use to communicate is not sound or words like humans, but advanced biological methods designed for efficiency.
By learning what do ants use to communicate, we realize why these tiny insects can achieve such large-scale cooperation.
Ants Talk to Each Other Constantly
It’s fascinating how often ants talk to each other while working. Every ant has a job, and constant interaction keeps the whole colony in sync. Imagine if thousands of ants worked silently, without signals—it would be chaos.
Because ants talk to each other using multiple strategies, they can adapt quickly to challenges like floods, food shortages, or predator attacks. This makes their survival strategy nearly flawless.
So, the reason ants talk to each other is clear: communication equals survival.
Conclusion: Why Do Ants Communicate?
In summary, why do ants communicate is one of nature’s most interesting mysteries. These tiny creatures communicate to share food sources, defend their colony, and ensure every ant knows its role. By studying what do ants communicate with each other, why do ants talk to each other, what do ants talk about, and what do ants use to communicate, we learn how powerful teamwork can be.
Ant colonies remind us that even the smallest beings rely on unity and connection. Just as ants talk to each other to thrive, humans also depend on communication for survival and growth.

Informational
Want to Become an Investigative Journalist? Start Here

Want to Become an Investigative Journalist? Start Here – A Complete Guide
If you’ve ever thought, “Want to become an investigative journalist? Start here,” then you’re already on the right track. Investigative journalism is one of the most impactful forms of reporting, uncovering hidden truths, exposing corruption, and giving a voice to the voiceless. But where do you begin? This article will guide you step by step on what it takes to build a career in this powerful field.
Why Investigative Journalism Matters
When people ask, “Want to become an investigative journalist? Start here,” they usually have one thing in common: a passion for truth. Investigative journalism isn’t just about writing stories; it’s about holding the powerful accountable. From exposing political scandals to uncovering human rights violations, investigative reporters play a crucial role in protecting democracy.
Skills You Need to Succeed
If you want to say confidently, “Yes, I want to become an investigative journalist. Start here,” then mastering a few essential skills is key:
Critical Thinking – The ability to connect facts, analyze patterns, and dig deeper.
Strong Writing Skills – Crafting compelling stories that inform and engage.
Research Mastery – Knowing where to find credible information and verifying facts.
Persistence – Investigations can take months or even years.
Ethics & Integrity – Upholding truth without bias.
Education and Training
Many people searching “Want to become an investigative journalist? Start here” often wonder if a degree is required. While a journalism or communications degree helps, it’s not the only path. Online courses, internships, and mentorships provide hands-on experience. Universities worldwide now offer specialized investigative reporting programs, while digital platforms give aspiring journalists real-world opportunities to publish their work.
Tools of the Trade
To move from interest to action, remember: “Want to become an investigative journalist? Start here” also means equipping yourself with the right tools:
Data Analysis Software (Excel, SQL, Python)
Freedom of Information Requests for accessing government records
Interview Techniques to extract accurate insights
Digital Security Tools to protect sources and data
Starting Your First Investigation
If you’re saying, “I want to become an investigative journalist. Start here,” your first investigation doesn’t need to be huge. Begin with local stories—community issues, workplace conditions, or environmental concerns. Build credibility by uncovering stories that matter to everyday people.
Building a Portfolio
Editors love proof of ability. Anyone typing “Want to become an investigative journalist? Start here” should immediately begin building a portfolio. Publish articles online, start a blog, or contribute to local outlets. Every story you write sharpens your skills and strengthens your credibility.
Networking and Mentorship
No journalist works in isolation. When you think, “Want to become an investigative journalist? Start here,” remember to connect with professionals already in the field. Join journalism associations, attend workshops, and reach out to mentors who can guide you through the complexities of investigations.
Challenges You’ll Face
The journey isn’t easy. Saying “Want to become an investigative journalist? Start here” also means preparing for obstacles like:
Legal threats
Difficulty accessing information
Financial constraints
Safety concerns
However, with persistence and proper training, these challenges can be overcome.
The Future of Investigative Journalism
The phrase “Want to become an investigative journalist? Start here” has never been more relevant. With digital media expanding, new opportunities are opening up for independent investigative reporters. Podcasts, YouTube channels, and online news platforms give aspiring journalists direct access to audiences without traditional gatekeepers.
Final Thoughts
If you’ve ever wondered, “Want to become an investigative journalist? Start here,” this guide is your first step. Success requires passion, persistence, and integrity, but the reward is making a real impact on society. Start small, build your skills, and never stop asking questions—because the world needs more truth-seekers like you.

-

 Business6 months ago
Business6 months ago☕️The Ultimate Coffee Lover’s Guide: From Caffeine in a Cup of Coffee to Turkish Coffee Delights
-

 Business6 months ago
Business6 months agoIn today’s fast‑moving economy, what industry is slowly dying and why?
-

 Lifestyle6 months ago
Lifestyle6 months ago🐦 The Ultimate Bird Watching Guide: Best Birdwatching Locations & Tips for Beginners
-
 Business6 months ago
Business6 months agoThe Many Faces of “Future”: Markets, Farming, Music, AI, and a Better Tomorrow
-

 Entertainment5 months ago
Entertainment5 months agoBlack Phone 2′ Cast Revealed: 5 Stars Return, 5 Actors Join & 1 Star Exits
-

 Tech6 months ago
Tech6 months agoUltimate Secret Android Apps You’ll Actually Love
-

 Health6 months ago
Health6 months agoA Journey Through Life’s Firsts: Health, Gaming, and Experiences That Shape Us
-

 Lifestyle6 months ago
Lifestyle6 months agoWhat is life Philosophy?



